Just because two birds look alike doesn’t mean they are closely related.
Most bird studies in the Neotropics focus on the Andes or the Amazonian Basin. A logical choice given the extraordinary biodiversity there. However, ornithologists have also started exploring Central America and Mexico (often referred to as Mesoamerica). A recent study on the White-collared Seedeater (Sporophila torqueola) complex is an interesting addition to this list.
Some Taxonomy
Most taxonomic authorities divide this species complex into three to five geographically restricted subspecies, which mainly differ in male plumage. In the Pacific lowland of Mexico you can find the subspecies torqueola. The Caribbean side of Mexico houses another subspecies morelleti. Finally, the subspecies sharpei occurs on the coastal Gulf of Mexico. To recap, here are the three subspecies accompanied by a map showing their distributions.
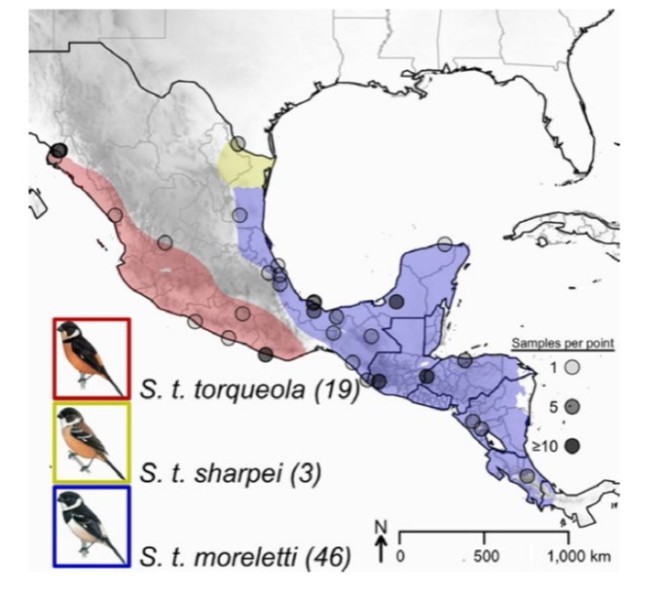
Distribution of the three Sporophila torqueola subspecies (from Mason et al., 2018)
A Lot of Specimens
Nicholas Mason and his colleagues measured over 600 specimens (although if you check the authors contributions, it turns out Arturo Olvera-Vital did all the measuring. Well done, Arturo!). The morphological characters clearly showed three clusters, corresponding to the three subspecies.
UCEs
For a genetic perspective, the researchers used UCEs (which stands for Ultra-conserved Elements, not Uganda Certificate of Education). These are a class of highly conserved and abundant nuclear marker distributed throughout the genomes of most organisms. The analyses of these markers revealed a deep split between torqueola and the two other subspecies. Despite their genetic differences, a demographic analysis indicated low levels of gene flow. There might have been some hybridization in the past.

A White-collared Seedeater (Sporophila torqueola) on the lookout (from http:www.hbw.com/)
No Sister Species
In addition, the mitochondrial DNA showed that these subspecies aren’t even closely related. The subspecies torqueola belongs to a clade with Grey Seedeater (S. intermedia) and Black Seedeater (S. corvina), whereas the subspecies moreletti belongs to a large group of species that include Slate-colored Seedeater (S. schistacea) and Plumbeous Seedeater (S. plumbea). So, although these species look alike, they belong to different groups within the Sporophila radiation.

A Black Seedeater (S. corvina) foraging (from http://www.hbw.com/)
References
Mason N.A., Olvera‐Vital A., Lovette, I.J. Navarro‐Sigüenza, A.G. (2018) Hidden endemism, deep polyphyly, and repeated dispersal across the Isthmus of Tehuantepec: Diversification of the White‐collared Seedeater complex (Thraupidae: Sporophila torqueola). Ecology and Evolution, 8(3):1867-1881.
This paper has been added to the Thraupidae page.







